More on Probiotics

Probiotics are thought by most people to be quite similar to vitamins, and considered effective and safe. But are they?
In my article on The ABCs of Gut Bacteria, we covered the classification of living organisms also known as ‘taxonomy’ which was devised by the Swedish biologist Carl Linnaeus. In our lovely tree of life species is furthest along the branch. But did you know that species themselves can contain multiple subgroups which we can visually view as little twigs off the species branch? These subgroups are called strains and are relevant to microbes.
Put simply a strain is a genetic variant of a microbe. For example, you may hear on the news about a particular strain of the flu virus causing people in the region of the world to get sick. These flu strains can be distinguished from one another by the type of protein found on their surface.
Just like viruses bacteria can also be distinguished by their genetic makeup. There is a special type of analysis called metagenomics which is able to analyse genomes in an environmental sample. Using this type of analysis we can work out the different types of bacterial strains in that sample.
When it comes to producing bacterial strains particularly commercially, a strain takes on a slightly different meaning. The definition of a strain becomes the “descendants of a single isolation in pure culture”. A single isolation in pure culture means that the strain has been isolated from a particular site e.g. vaginal flora, and then grown and replicated in an artificial culture.
Probiotic history
In talking about probiotics I’d like to tell you stories of some of the key influential figures in modern probiotic history starting from the founding father Elie Metchnikoff.
Elie Metchnikoff was born in Kharkiv, Ukraine in 1845 and under the influence of his mother who was primarily responsible for his education in early life he developed a love for science. He was encouraged by his mother to pursue natural sciences and academia rather than medicine and this proved to be a very good decision. If you were hiring Elie Metchnikoff for an academic appointment you could not fail to see that he was a research prodigy and made the most of opportunities that came his way.
At the age of 17 he enrolled in Kharkiv University for the natural sciences and completed a 4 year course in 2 years. Biology researchers often do a lot of travel and he spent the next few years in Germany and Italy training in comparative anatomy. Comparative anatomy is the study of similarities and differences in the anatomy of species. By the time he was 22 years of age he had made important discoveries on the embryonic development of cuttlefish and Crustaceans, uncovered the process of intracellular digestion in a flatworm and won a major prize with co-investigators for research in the development of germ layers in invertebrate embryos.
It was at the age of 22 that he was appointed Docent (an equivalent academic title for Academic Professor) at Ukraine’s Odessa University where he was younger than many of his students.

Three years later at the age of 25 he was appointed full Professor of Zoology and Comparative Anatomy at Odessa University. Years later when Metchnikoff was working in Messina, Italy he noted when studying the digestive organs of starfish larvae that particular cells not related to digestion were enveloping and devouring dye particles that he had introduced into the larvae.
Advice from a zoology colleague Professor Carl Claus enabled this process to be termed phagocytosis which is derived from the Greek terms of ‘phagein’ (eating) and ‘klytos’ (cell). Metchnikoff published his research findings in a paper “Intracellular digestion in intervebrate animals’ and came to realise that this process of phagocytosis was critical in protecting the host animal against infection.
His research work led him to a job at the Pasteur Institute laboratory, Paris in 1888 and he became the deputy director in 1904 where he continued his research on phagocytosis. For his research work in showing how specific white blood cells break down harmful bacteria in the body through phagocytosis Metchnikoff was awarded the Nobel Prize in Medicine in 1908 along with Paul Ehrlich (who as we know discovered mast cells and basophils).
A very common saying we use is “Don’t rest on your laurels” which means that we shouldn’t just be satisfied with our past successes and not strive to do anything more. This saying originates from laurel wreaths that were crowned to victors of the Pythian games at Delphi. A ‘laureate’ was originally a person crowned with a laurel wreath. Nowadays our most famous laureates are Nobel laureates who receive a medal, diploma, cash award and of course massive international recognition and acclaim.
After an achievement as great as winning a Nobel prize it might be perfectly fine to rest on your laurels but Metchnikoff had other more important considerations. He wanted to save humanity. The year before he was awarded the Nobel Prize he was working in Bulgaria. He was intrigued as to why certain members of the Bulgarian population would live much longer than others. Specifically, he was interested in those that lived beyond the age of 100 or as centenarians as we would call them. He found that these poor villagers living in the Caucasus mountains were as part of their simple lifestyle consuming a fermented yoghurt drink on a daily basis. Metchnikoff linked this to the “Bulgarian bacillus” which had been discovered in natural yoghurt in 1905 by a 27-year-old Bulgarian physician Stamen Grigorov. This lactic acid producing bacterium is now known as Lactobacillus bulgaricus.
Metchnikoff believed that the colon was the source of “toxicants” or toxic substances that contributed to illness and ageing. He also believed that replacing our native harmful colonic microbes with friendlier and useful microbes would be helpful for human health. In testing this hypothesis Metchnikoff drank sour milk every day of his life. He was apparently “well pleased with the result” but we have no idea what exactly this meant. It was only with the advent of clinical trials that we can begin to quantify the health benefits of these administered live bacteria. We call these administered live bacteria which when conferred in adequate amounts give a health benefit to the host probiotics.
Metchnikoff died at the age of 71 not having reached his goal of being a centenarian but most certainly leaving a legacy in the history of medicine.
At the time of Metchnikoff’s promotion of the health benefits of lactic acid bacteria, Henry Tissier, a French paediatrician who also worked at the Pasteur Institute found that infants with diarrhoea in their stools had a low number of a Y-shaped bacteria. In contrast, there were a large number of these “bifid” bacteria observed in the stools of healthy breast-fed infants. Tissier suggested that these bacteria which we now know as Bifidobacterium bifidum could be given to babies suffering from diarrhoea to restore their healthy gut microbe population. His administration of Bifidiobacterium bifidum to treat infantile diarrhoea is very likely the first oral administration of a live microbe to specifically treat a disease.
Occasionally we come across a chance discovery in a patient that can revolutionise the course of medicine. A good example of this is the story of Henrietta Lacks an African American lady whose cancer cells are the source of the immortal HeLa cell line. These cells have the ability to reproduce indefinitely under specific laboratory conditions. They have been and still are monumentally significant for cancer research.

The German physician and bacteriologist Alfred Nissle came across such a discovery in a patient in 1917. At that time, it was World War 1 and disease was rife particularly in the Balkans that had been hit hard with an epidemic of gastroenteritis caused by Shigella. Alfred Nissle came across a soldier in a military hospital near Freiburg, German who had not developed any diarrhoea during his time in the Balkans. He found a particular strain of E.coli from the soldier’s stools that he cultivated and discovered in the laboratory that it could inhibit the growth of many other gut pathogens. This raised the prospect that certain live bacteria have (as part of their beneficial function) the ability to directly fight off other harmful microbes. This strain of E.coli was later named the E.coli Nissle 1917 and is still in commercial use today as a probiotic.
Growing up in the early 20th century in rural Japan mean that Minoru Shirota had witnessed firsthand the direct effects of poor hygiene and outbreaks of food borne illnesses. He entered medical school at Kyoto University with the aim of preventing these transmissible diseases. He developed a real interest for microbiology inspired by the research of Elie Metchnikoff.
Although Elie Metchnikoff was a great believer in the health properties of lactobacillus bulgaricus for the human gut, subsequent research work found that this bacterium in fermented milk did not survive exposure to gastric acid in the stomach. Therefore, there was understandably a great deal of doubt about the benefits of introducing live bacteria to the human gut. In 1930 while working at the microbiology laboratory in Kyoto University Minoru Shirota was able to cultivate a strain of lactic acid bacteria that could survive passage through the stomach and colonise the small bowel and colon. The strain that he discovered is now known as Lactobacillus casei strain Shirota.
Shirota patented the discovery and was able to manufacture a fermented drink containing the bacterial strain that he released under the Yakult trademark in 1935. Many of you will have heard of Yakult and are familiar with the small 65 ml bottles that we see at supermarkets. It was really after Japanese consumers started to embrace the benefits of Yakult that the manufacturing and marketing of the product went global. Domestically the marketing was very successful in large part due to the Yakult ladies who wore a particular distinctive attire and carried out door-to-door sales of Yakult across the country. They’re still very visible and active to this very day. This all goes to show that a product may itself be intrinsically great but the marketing has to be even better for it to be a commercial success.
Lactobacillus and bifidobacterium species are the most commonly used probiotics but a lot of other probiotic bacterial strains have been discovered. See Figure 1.
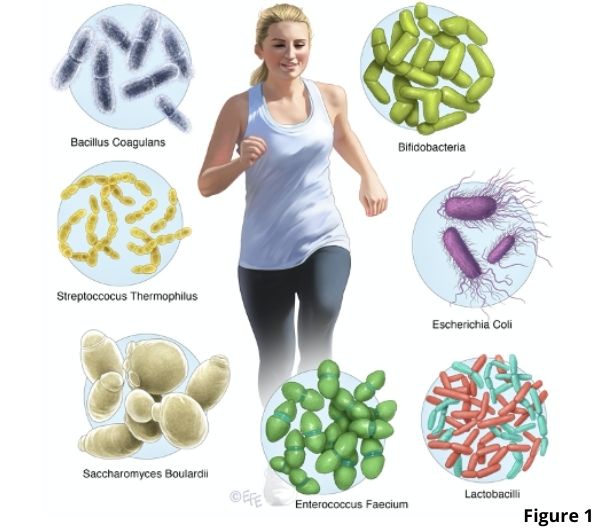
Many of the commercial probiotic strains originate from the intestine of healthy infants and adults. A probiotic that is not found naturally in the human gut is a yeast called Sacccharomyces boulardii. This yeast is closely related to baker’s yeast used in making bread and owes its discovery to the French scientist Henry Boulard.
During the cholera outbreak in IndoChina Boulard observed that some people who chewed the skin of lychee and mangosteen (or prepared a special tea from them) did not develop cholera symptoms. He successfully isolated the yeast in 1923 and it is named after him.
Even though it is not a bacterium Saccharomyces boulardii is in many ways a perfect probiotic. It is able to survive the effects of stomach acid and bile released in the small bowel. It can also adhere to the intestinal mucosa where it colonises.
Adhesion is an important property for a probiotic because you don’t want the microbes to be easily flushed down the toilet with stool. Adhesion also means that the microbes can provide a protective lining of the intestinal epithelium and prevent other harmful microbes from attaching. A more recent concern regarding probiotics is the potential to transmit antibiotic resistant genes given the overuse of antibiotics in modern society. No transfer of genetic material occurs between yeast and bacteria making it safe to use during antibiotic treatment. Saccharomyces boulardii also grows very well at human ambient body temperature.
Yeasts like Saccharomyces and of course bacteria have been used in fermentation for a very long time. Fermentation is a process where sugars such as glucose are converted to other compounds like alcohol in an oxygen free environment. Bacteria and yeast are the microbes used in this process with lactic acid generated in the case of bacteria and alcohol by yeasts.
It’s believed that the first people to carry out fermentation were the Natufians, a group of Stone Age hunter-gatherers who occupied what is now modern-day Israel and Jordan. Researchers have discovered the oldest known alcoholic beverage (beer) dating back 13,000 years at a Natufian burial place in Israel. The Natufians made and consumed beer as part of their ritual feast in honour of the dead. The Natufians very likely made the first bread with evidence of an ancient pita-like flatbread uncovered in the Black Desert in Jordan. Yoghurt itself is believed to have originated from ancient Mesopotamia around 5,000 B.C.
It is quite amazing to find that so many cultures around the world have their own fermented foods. For example, kimchi originally comes from South Korea, kombucha from Russia and China, sauerkraut from Germany, sourdough from Egypt, miso from Japan, dosa from India, cortido from El Salvador, and there are many others. The widespread use of fermented foods and beverages is a reason why many people rave about taking their own ‘natural’ probiotic. They understandably feel that all the positive health benefits that have been found on clinical studies for probiotics can be achieved through their own consumption of their favourite fermented food or beverage.

But yoghurt and other fermented foods are not technically probiotics. They are considered functional foods. Probiotics are reserved for products with an adequate number of microbes that can confer health benefits for humans in controlled clinical studies. This means that there are a lot of fermented foods that miss out on being classed as a probiotic but there are a small proportion which have been studied and shown to have a probiotic effect.
Probiotics can come in many different formulations. The most common delivery route is orally through the use of tablets or capsules. They can be added to yoghurt, fermented milk and even cheese. Some forms of probiotics that are freeze-dried can be packaged without the need for refrigeration. Probiotics can be made as a gel for vaginal use or as a cream to be applied on the skin.
As a gastroenterologist I am asked about probiotics all the time by patients. One of the most common questions is about how long to take the probiotic for. The answer to this really depends on the clinical reason for why you’re taking the probiotic. If probiotics are being used to treat antibiotic associated diarrhoea then it would be only for a short period lasting the duration of symptoms and a couple of days afterward. For a chronic condition such as ulcerative colitis or irritable bowel syndrome probiotics can be used in the longer term. The effects of probiotics on the gut appear to be temporary and disappear with discontinuation of the probiotic. We can detect the presence of probiotic bacteria in stool and once a probiotic is stopped, the bacterial presence disappears fairly quickly from stools. This also applies to the yeast Saccharomyces boulardii that is cleared from stools 2-5 days after stopping.
The other question I get asked a lot is what probiotic should I use? A very understandable question from patients given the sheer range of probiotics out there. It’s actually a good question to ask but rather difficult to give an answer.
The reason for this is that many clinical trials differ in their study design. There are differences between the strains used especially if they are commercial proprietary strains and drastic differences between the duration and dosage of probiotic use. Quite often probiotic strains can be mixed together or even combined with other compounds such as prebiotics. We’ll talk more on prebiotics in the next chapter.
Well-designed clinical trial information is very useful but there is a saying that 99% of statistics only tells 49% of the story. I am always cautious as a clinician about blindly applying the results of studies even from my favourite summary of clinical trial information – the meta-analysis. This is because the patient may have individual circumstances that are quite different to the population being studied in a clinical trial. For example, the ethnicity of your patient or their diet may not match the people that were studied in a clinical trial.

Having said all this my advice to patients again goes back to the specific clinical situation.
I will find the appropriate research data on probiotic use for their particular condition and suggest that based upon the relevant clinical studies that a particular probiotic strain(s) may be of benefit. I will always caution that it may not be helpful for their particular condition, and in no way should it replace standard medical treatment.
I do see the role of probiotics as complementary to standard medical care. Certainly, like vitamins probiotics are used widely and is now a multi-billion-dollar industry. As I alluded to before many ‘probiotics’ are actually functional foods and are not subject to the same regulation that drugs for example would be subjected to.
There is a lot of research however that has been done to evaluate the effects of probiotics for a whole host of medical conditions and these have been published in peer-reviewed journals. When it comes to making claims that probiotics can improve the health of children and especially infants the benchmark is much higher and stringent. Companies do need to prove in rigorous clinical trials that their product actually works.



