How the gut defends itself
The gut not only has the functions of digestion and absorption but increasingly we are appreciating its role in the immune system. It is the immune system that is responsible for detecting and destroying foreign harmful microorganisms.
The study of the immune system is ‘immunology’ and this is becoming an increasingly complex medical field as we learn more and more about the underlying mechanisms underpinning how our immune system works.
We’ll start with some key players in the immune system – T and B cells. These cells can be thought to be very highly specialised defender cells and are tailored to different microbes. If your body is infected with a particular microbe, only the T and B cells that recognise it will respond. These selected cells then rapidly multiply and form an army of identical cells or clones that will fight the infection. Some T and B cells can remember the microbes
that caused the infection and this leads to protective immunity.
B cells produce proteins called antibodies that can target the microbes whilst T cells directly attack and kill microbes. Both T and B cells recognise the microbial invaders by the shape of molecules called antigens that are present on their surface. Your immune system can provide a T and B cell to lock onto every possible antigen. When the baby is developing in the womb the T and B cells that recognise certain patterns in the body as potentially foreign were destroyed to prevent them from attacking the body. This is an important mechanism known as clonal deletion that is central in preventing autoimmunity i.e. the body’s immune system attacking itself. This discovery was first made by the Australian Sir Frank Macfarlane Burnet and he was awarded the Nobel Prize in Medicine in 1960 in recognition of its significance.
There are however millions of T and B cells remaining that can recognise and target every foreign antigen that your body might encounter.
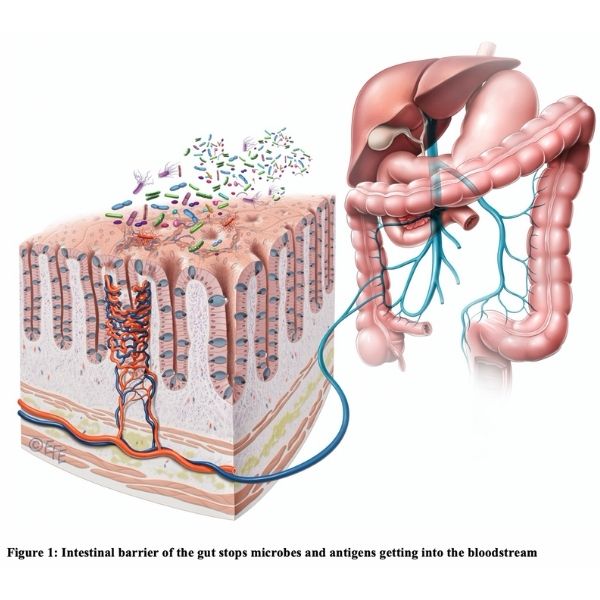
Another important immune defence that our body has against microbes is the epithelium. In the gut the epithelial cells are joined together via tight junctions and serves as a protective barrier (see Figure 1) to prevent microbes and antigens from getting into our blood circulation.
At this point I want to bring up a story of mine which turned out to be a salutary lesson. A patient once presented to my office saying that he felt a leaky gut may have caused some of his health issues. He was very passionate in his belief that a leaky gut was relevant to his health. This was before I knew much about the role of the gut in the immune system and so I dismissed this as a fanciful theory. I then had to eat humble pie and apologise profusely to the patient later when I came across reams of medical literature that essentially described the phenomenon of the leaky gut but used the medically sanctioned term ‘intestinal permeability’.
The leaky gut concept arises from the idea that when the gut is unhealthy the lining can weaken and ‘holes’ can develop in the epithelial barrier. This then allows toxins and microbes to leak into the bloodstream. A whole host of inflammatory and immune reactions can ensue and potentially affect health.
These ‘holes’ may be due in some cases to genetics or in other cases it could be the influence of the environment such as eating an unhealthy diet that is low in fibre and high in saturated fats and sugar. Age is likely to play a role since the older you are the more easily your cells can get damaged and healing is slower.
There is research that this leaky gut or intestinal permeability is linked to a higher risk of autoimmune diseases such as lupus or multiple sclerosis. People are looking at the role of the leaky gut in chronic fatigue syndrome. It is still hard to measure the leakiness of the gut although there are biomarkers such as LPS-binding protein. Intriguingly recent research was carried out on 43 healthy married couples discussing and attempting to resolve a challenging conflict. Higher levels of LPS-binding protein were found in the blood samples of couples that argued more compared to the couples that were calmer. The research was published in the journal Psychoneuroendocrinology and when I mentioned this to my wife one night she simply said ‘happy wife, happy life’. There is something perhaps to that old adage.

For me the learning lesion with my patient was to never discount anything that is said to you in earnest relating to one’s health no matter how absurd it might seem at the time. There is simply too much in the world that we still don’t know about.
At this point I’m going to bring up a question that’s frequently brought up by my medical students. We know that the immune system recognises and defends very well against foreign microbes such as bacteria, viruses and fungi. Why then does the immune system not attack all of the microbes that are found in our gut? Surely they are foreign to our body and thus we would expect an immune system to mount a powerful response to eliminate them from the gut?
In order to answer this question we must recognise the special mechanisms that our body has developed to protect our gut microbiota while protecting the rest of the body against foreign microbes and antigens.
The best way to conceptualise this explanation is to think of the immune system in the gut as a battleground. This is a fantastic battleground so please indulge me for bringing the occasional mythical creature into the battlefield.
Let’s imagine an invading army which is just outside a castle. We’ll visualise this in Figure 2.
These marauders represent foreign microbes. They have a distinct coloured pattern over their armour. This pattern represents an antigen.
The castle wall represents the epithelial cells of the gut. Beyond the castle is the gateway to the kingdom and it’s vital that the invaders be prevented from entering the kingdom. However we are happy with the marauders staying outside the castle as they do provide a valuable source of nutrients to the castle inhabitants.
The marauding army is massive but the good news is that there are some natural or innate defences that the castle possesses. Firstly it is surrounded by a protective moat. The moat differs slightly between castles of the small intestine and castles of the large intestine. The small intestine has only a single moat that is made up of gelatinous mucus that is created by special mucus cannons (goblet cells) mounted on the castle wall. The small intestine castle unlike the large intestinal castle has very good crossbowman (Paneth cells). They are able to reliably fire off crossbow bolts (antimicrobial peptides) at the invaders to keep them in check.
The large intestine castles however have 2 moats and they have a lot more goblet cells that can create and fire off mucus. The marauders can breach the thinner outer moat but rarely do they get past the inner moat which is very heavily covered in thick mucus.
Hidden in the mucus are large amounts of special traps called IgA antibodies that help trap invaders.
For both castles traditional cannons are mounted on the ramparts and deploy cannon balls (alpha and beta defensins) regularly to fire at the invaders.

You may at this point wonder how a castle can spot these invaders in the first place. Pattern recognition of these invaders is really important and the castle has a number of special watchtowers with sentinel guards. A particular watchtower is called a Toll-like receptor and you may think as containing a large bell that is rung to alert the castle to start the firing of antimicrobial peptides and production of IgA antibodies.
Once the bell is tolled messages (cytokines) are dispatched to other castle defenders telling them that a battle is afoot.
These innate defences of the gut are good but they’re not all that specific. In many ways it reminds me of the arcade game Space Invaders. It was an eon ago when I played
this game but I still remember vividly trying to shoot at enemy ships before being completely overwhelmed by the armada descending upon me. The key point here is that if you want something more effective against the marauding army then it’s time to get all the troops assembled.
So it’s time to adapt to the reality that more specific defences are required against the invaders. If you’re a vertebrate, or in other words if you possess a backbone, adapting and refining your immune defences is the way to go. Let’s bring in our new defenders.
Dendritic cells are found in both large and small intestine castles and pictorially they can be represented by the mighty giant squid-like creatures called Krakens.
These have long tentacles that can reach over the castle and feed on an invader as it’s stuck in the thick mucus of the inner moat. You may be surprised to hear that in each of these tentacles is a small bell that tolls when it senses the pattern of an invader. You’d be 100% correct if you said it was a mini toll-like receptor. Now the tentacles rip off a section of an invader’s antigen armour which is swallowed by the Kraken. The Kraken processes that antigen internally and presents small bits of it to its other tentacles which it uses to tap other castle defenders to spur them into action.
Important castle defenders that dendritic cells tap are B cell longbow archers that shoot arrows (antibodies) at their targets. Key castle defenders are T cell knights that fight in direct hand to hand combat. Some of the T cell knights wield flaming swords that can heat the place up (pro-inflammatory). Too many of them all at once can burn the whole castle so they have to be tempered by a few other T cell knights (regulatory T cells) wielding swords of ice.

Some T cell knights are great teachers or master-at-arms. They are especially good at getting the B cell archers speedily through their training academy and prepared for battle. These wise old teachers are called helper T cells.
B cell archers and T cell knights have been specially chosen from birth to protect the castle and kingdom. There are a great many of them and each of these B cells archers and T cell knights can recognise a particular invader. If an invader army (infection) arrives the matching B cell archer and T cell knights that recognise them can clone themselves very quickly. After the battle is won many of the clones will die off as they are no longer needed. A small number around 10% however do end up surviving long term as castles and kingdoms need their collective battle wisdom. They become revered as warrior
monks. Some will spend their time residing in castle libraries (gut) where they write up their memoirs whilst others travel around the kingdom spending time with squadrons of B cell archers and T cell knights (lymphoid tissue). Warrior monks are also known as memory B and T cells and are not averse to battle. If an invader army that they’ve fought off before returns to attack the castle – memory B cells fire off far more arrows (antibodies) than they did before. Memory T cells are much more potent and vigorous in combat.
The castle walls are among its strongest defences. But this can be breached when holes are created in the wall itself and small invaders creep through. Once the invaders breach the wall (epithelial layer) they arrive at the main courtyard of the castle where a fierce battle takes place. The large trolls of the castle (macrophages) take the opportunity to gobble up the invaders. Like the Krakens they have the ability to rip off a section of an invader’s antigen armour, process it in their strange bellies and then present small bits of that hide dangling on the ends of their twisted gnarly arms. Almost as if they want to show these antigens off as hideous trophies. The ends of their arms just like the Kraken’s tentacles also contain little bells that toll when it senses the pattern of an invader. Perhaps the innate harmony orchestrated by these toll-like receptors in some way counterbalances the monstrous visage of these mythical defenders.
The monstrous trolls are loyal to the castle and waving their gargantuan arms dangling little bits of antigen they prime B cell archers and T cell knights for combat. Sometimes when they flail their arms around they can tap whole squadrons of T cell knights and B cells archers for imminent battle.
These squadrons have arrived at the castle grounds through the waterways of the kingdom. These squadrons are symbolic of lymphoid tissue that has lots of B and T cells. The waterways represent the lymphatic system. The gut interestingly possesses the largest mass of lymphoid tissue in the body.
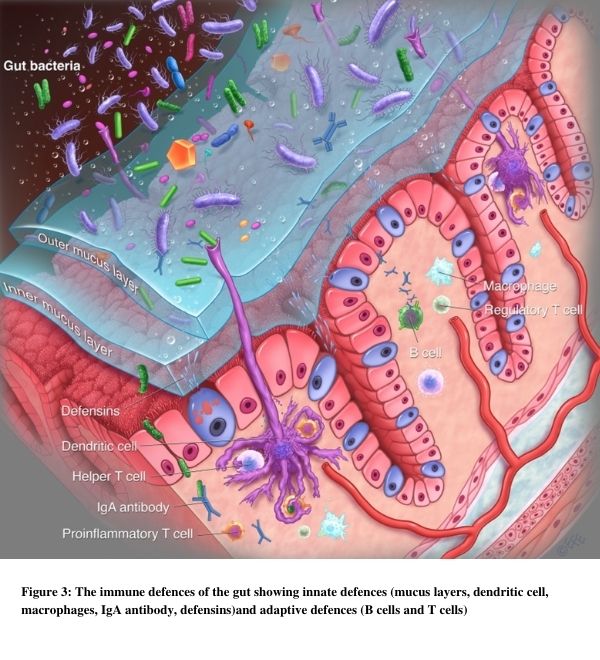
In our analogy we can see that there are 2 main methods to contain the gut microbiota army. The most important one is the physical separation of gut bacteria (invaders) from areas of immune activity (castle). The mucus layers and epithelial cells with tight junctions help to achieve this along with the surveillance network of the dendritic cells.
When bacteria do get past the wall there is an adaptive immune response that is contained (castle grounds) so as to prevent the body’s widespread immune activation (microbes entering the kingdom). Macrophages and lymphoid cells help to attack and destroy microbial invaders.
The state of war represents an ongoing equilibrium that enables both the gut microbiota to survive and flourish as well as preventing an unhealthy widespread immune response. If our castle defences were to mount an aggressive response and go out to attack and completely overwhelm the invaders so as to destroy them
– it would rid us of all our own protective gut bacteria. This is of course useful when we need to combat harmful microbes in nasty infections. However when it comes to our resident gut microbes (also called commensals) our immune system learns to recognise them and keeps them in the state of equilibrium described. See Figure 3.



